What is grease? It’s a solid to semi-fluid product made up of a liquid lubricant and a thickening agent. The type of thickener and the characteristics of the liquid lubricant determine the basic properties of a grease.
Grease is made up of three components:
Base Oil (70-90%) + Additives (1-10%) + Thickener / Soap (5-20%) = Lubricating Grease
Base Oil
Oil is the primary component of grease and is trapped by the thickener to make it denser or stiffer. Base oils can be either mineral oils or synthetic fluids. Most greases use mineral oil, though in temperature extreme circumstances, a synthetic base would be used. The lubricating properties of the grease come from the oil along with the additives, not the thickener. However, the thickener does affect the performance and the suitability of the grease application.
Since base oil does the lubricating, viscosity is still the most important characteristic. The following are grease viscosity levels and applications:
- ISO 100 & lower: Used for high speeds >3600 rpm, lower loads, good at low temperatures
- ISO 150/220: Moderate speeds up to 3600 rpm, good load carrying, typical multi-purpose grease’s oil
- ISO 460: Higher loads than ISO150/220, often with improved water resistance
- ISO 1500: Typical speeds <100 rpm, excellent load carrying, good water resistance
Additives
Additives function as several roles in a lubricating grease, including:
- Enhancing existing desirable properties,
- Suppressing existing undesirable properties
- Imparting new properties.
The most common additives are oxidation and rust inhibitors, extreme pressure, anti-wear, and friction-reducing agents. In addition to these additives, boundary lubricants such as molybdenum disulfide (moly) or graphite may be suspended in the grease to reduce friction and wear without adverse chemical reactions to the metal surfaces during heavy loading and slow speeds.
Thickeners
Thickeners are comprised of either:
- Soap base (chemical structure similar to soap)
- Non-soap base
Shear stability, load carrying capacity and higher working temperature are improved with complex greases
- Simple soap thickeners are formed by reacting a metallic hydroxide, or alkali, with a fat, fatty acid, or ester.
- A complex soap is formed by the reaction of an alkali with a high molecular weight fat or fatty acid to form a soap, and the simultaneous reaction of the alkali with a short chain organic or inorganic acid to form a metallic salt (complexing agent.)
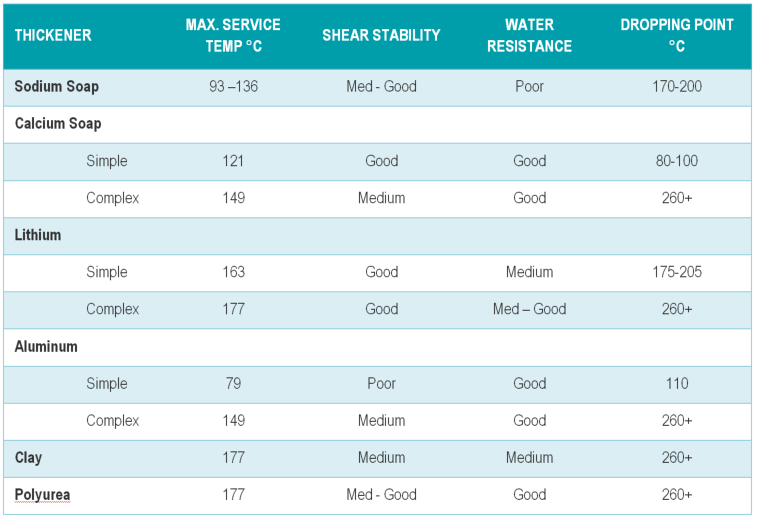
Polyurea, Organophillic Clay, and other non-soap greases make up about 11% of greases. All others are soap-type. Lithium and Lithium complex are the most common by far – 68% in North America.
- If the thickener ends in ‘-ium’ then it is a soap – lithium, calcium, sodium, aluminum (four most common). The grease with the best water resistance is Barium and aluminum complex. Whereas the worst water resistance is a sodium grease.
- Polyurea is often used in electric motor-bearing applications.
- Organophillic clay is used in some high temperature non-melting greases.
Thickeners have an effect on the following aspects of a grease:
- Temperature range
- Pumpability
- Water resistance and stability (Grease are able to absorb water and still retain their lubricating effectiveness)
- Mechanical stability of the lubricant
Below is a chart on the basic properties of each grease thickener:
As with any lubricant, we recommend that you consult the service manual or contact your machinery manufacturer to see what they recommend when determining what grease to use in your specific application.
If you have any questions or would like to learn about grease testing and analysis, contact a Fluid Life representative.
Article by Drew MacRae