Fuel Distillation (ASTM D86)
ASTM D86 is the standard test method used to determine the boiling range of a petroleum product. This is done by performing a batch distillation at atmospheric pressure resulting in a distillation curve. This standardized test is related to volatility and fuel performance for gasoline and diesel fuels.
Volatility
Volatility characteristics of gasoline and diesel fuel are different. Gasoline utilizes spark ignition while diesel ignition is based on compression. If the volatility of gasoline is low, it won’t exist in the “gaseous” phase and you would experience issues with start up and performance in cold climates. If volatility is high, gasoline can be vaporized in the gas tank itself or in the gas lines causing vapor lock. As a result, the fuel injection rate is low and the engine “drowns”.
For diesel fuel, if volatility is low, the higher the boiling point components and the higher combustion times and the poorer the combustion of heavy hydrocarbons. This will lead to the formation of smoke, soot deposits (which can obstruct fuel lines, injector nozzles, cylinder walls, intake valves and more), a loss of power and increased fuel consumption. If the volatility of the diesel fuel is high, this can cause vapor lock.
Fuel Distillation Test Use Cases
A universally accepted measure of the volatility of a fuel is the distillation test. As mentioned, ASTM D86 is the standard methodology for this test and measures the percentage of vaporized fuel as the temperature increases. For diesel engines, the use cases for the fuel distillation test are the identification of summer versus winter fuel and gasoline contamination in diesel fuel.
Summer vs. Winter Diesel Fuel
Winter blends supplied by fuel manufacturers are a mix of #2 and #1 diesel. Fuel normally used in the warmer months is #2 diesel and contains the liquid form of paraffin wax. The #1 diesel fuel is more refined, contains kerosene and less likely to gel in cold weather due to the lower pour-point and cloud point of kerosene. Summer diesel fuel is normally used from May to October while a winter diesel is used from November to April.
Running a winter diesel fuel in summer can cause a loss of power and increased fuel consumption because the added kerosene dilutes the fuel. If running a summer fuel in winter in colder climates, the fuel will thicken, get cloudy, and reduce flow rates through the fuel system. Eventually the diesel fuel will gel and stop the engine from running.
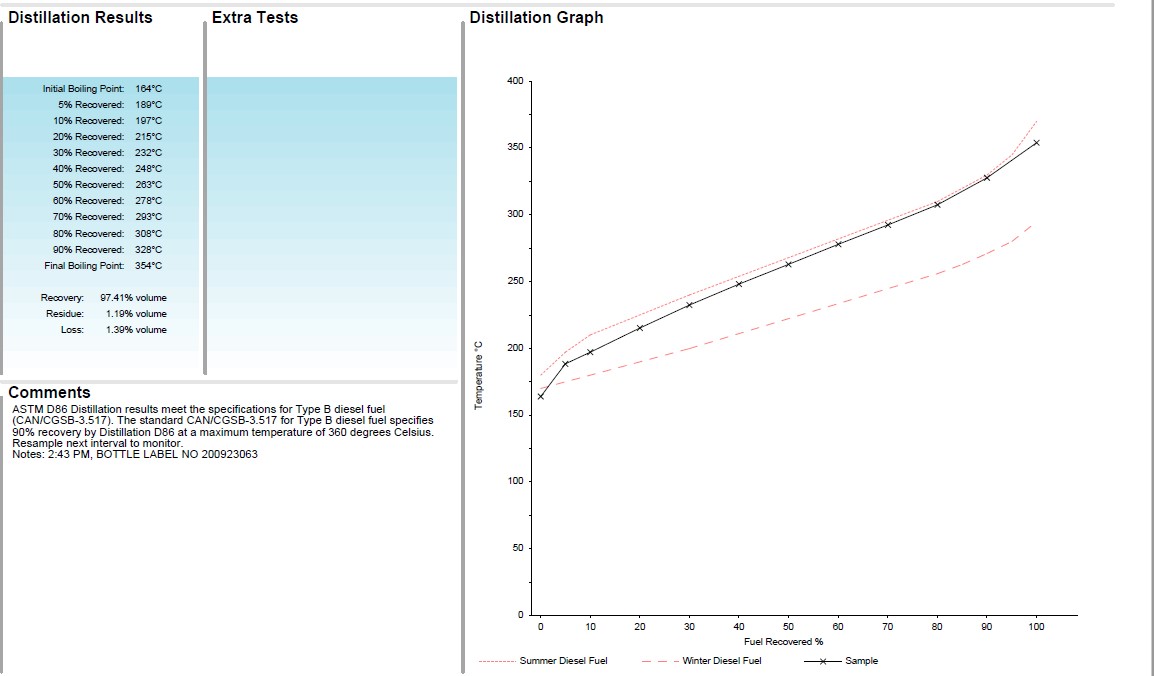
Distillation Curve of Summer vs. Winter Fuel
In the example below, the black line is the distillation curve of a diesel fuel sample and the two red lines are the Type A diesel (Winter Fuel, bottom) and Type B diesel (Summer Fuel, top) that are placed on a report as a comparison. In this case, the sample looks like a typical acceptable distillation curve for a diesel fuel meeting specifications for Type B Summer Diesel Fuel. Typically it’s somewhere in between the two. This test result can indicate if the correct fuel is used for the weather conditions and if a mix of the two has occurred.
Gasoline vs. Diesel Fuel
Compared to gasoline, diesel fuel’s flash point and ignition temperatures are significantly higher as it is heavier than gasoline and has a different density and viscosity. Gasoline, on the other hand is lighter than diesel fuel and has a lower flash point.
As little as 1% gasoline contamination in a diesel engine can cause a drastic reduction in the flash point of the diesel fuel. This lower flash point will cause the diesel fuel to prematurely ignite leading to engine damage. Gasoline contamination can also cause damage to the fuel pump and injectors due to the drop in lubrication properties of the diesel fuel as gasoline is a solvent. Additional issues related to gasoline contamination are incomplete combustion (black smoke), reduced power and performance, and increased soot buildup leading to significant engine damage.
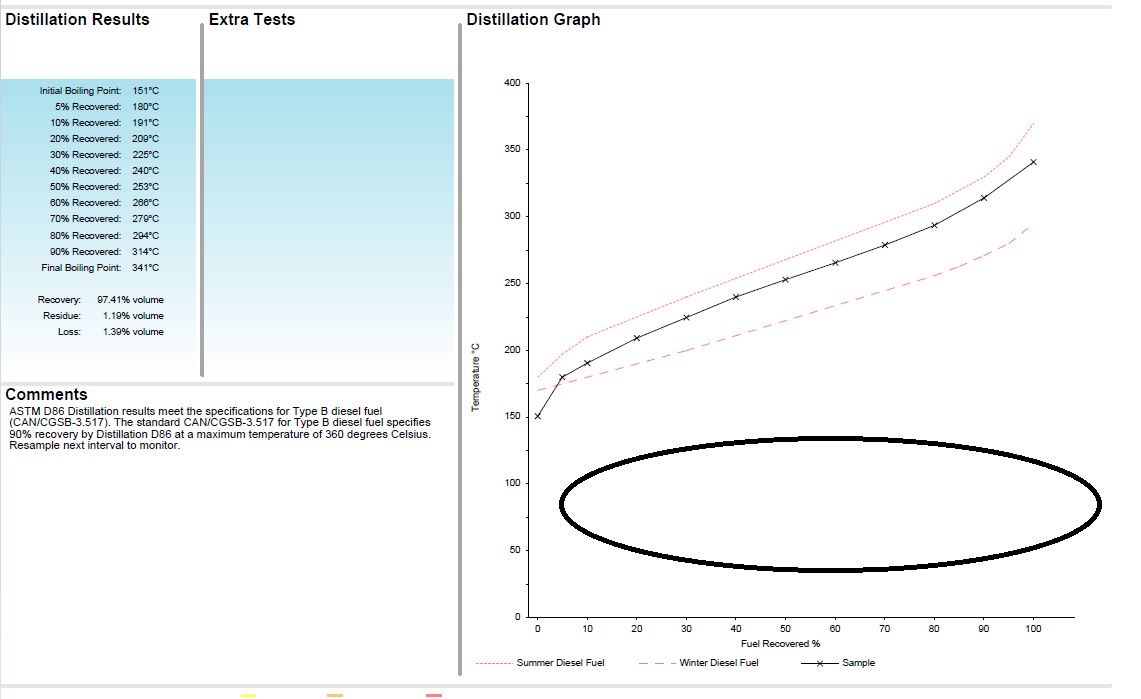
Distillation Curve of Gasoline vs. Diesel Fuel
Compared to diesel fuel, gasoline has a lower curve found in the circled area in the image below. If there is a mixture, then normally we would find a curve that sits somewhere in the middle.
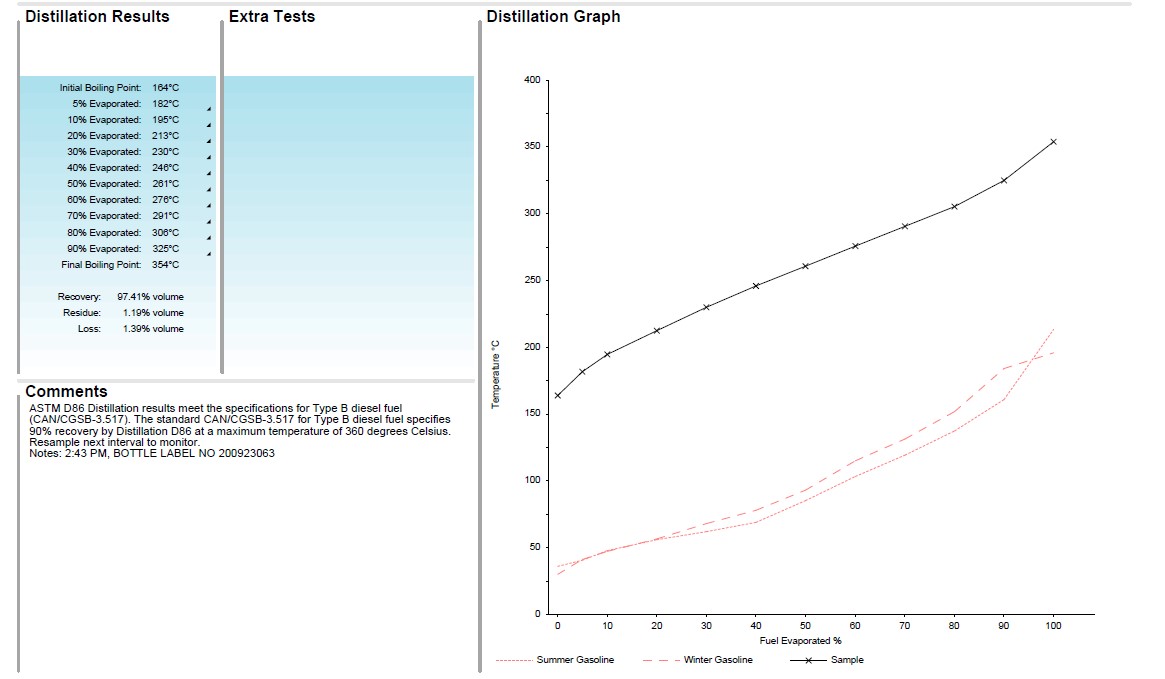
In the example below, this is a sample of diesel fuel (black line) compared against gasoline baselines (red lines). As you can see the gasoline distillation curves are much lower on the graph as the boiling points of gasoline are much lower than diesel. If there was any detectable amount of gasoline (or some other lighter substance) we would typically see some boiling points that are lower (how many and how low depends on the amount of contamination).
Additional Resources
- Diesel Fuel Testing and Analysis – overview of available diesel fuel tests and packages
- Blog: 4 Common Diesel Fuel Issues & Effects
- Blog: Baby It’s Cold Outside: Cold Weather & Diesel Fuel
- Video: Introduction to Diesel Fuel Analysis
- Download: Recommended Diesel Fuel Testing Packages
- Download: Diesel Fuel Individual Tests
